Eligibility
Your participation in this research study, that was approved by the Research Institute of the McGill University Health Center, will allow you to be screened for ovarian and endometrial cancer. All individuals, symptomatic or asymptomatic, between the ages of 45 and 75 who have a uterus are eligible to participate.
Please note that having a previous tubal ligation could affect the detection of ovarian cancer.
We will also be accepting younger individuals who are at risk of developing ovarian or endometrial cancer (inherited genetic mutation).
Read the Frequently Asked Questions section for more information.
For women and individuals aged 45+ who do not have a uterus but present with vague symptoms such as difficulty eating or feeling full quickly, bloating or swelling of the abdomen, abdominal discomfort or pain, vaginal bleeding, painful intercourse, increased urinary frequency and changes in bowel movements, please consult our other study DOvEE for rapid access HERE.
Procedure
Visit 1: sign a consent form and complete all study related procedures. This includes completing a questionnaire, collecting a saliva sample (2mL), a blood sample (5mL), a transvaginal ultrasound, a conventional pap-test and the DOvEEgene test. This visit should not last more than 1 hour and a half.
Visit 2: in-person follow up where a repeat blood test will be performed and the results of the DOvEEgene test given to you. This visit should not last more than 20 minutes.
Phone follow up: you will receive 3 phone calls from our team after your second visit to follow up and ensure that there are no new health issues.
Read the Frequently Asked Questions section for more information.
Location
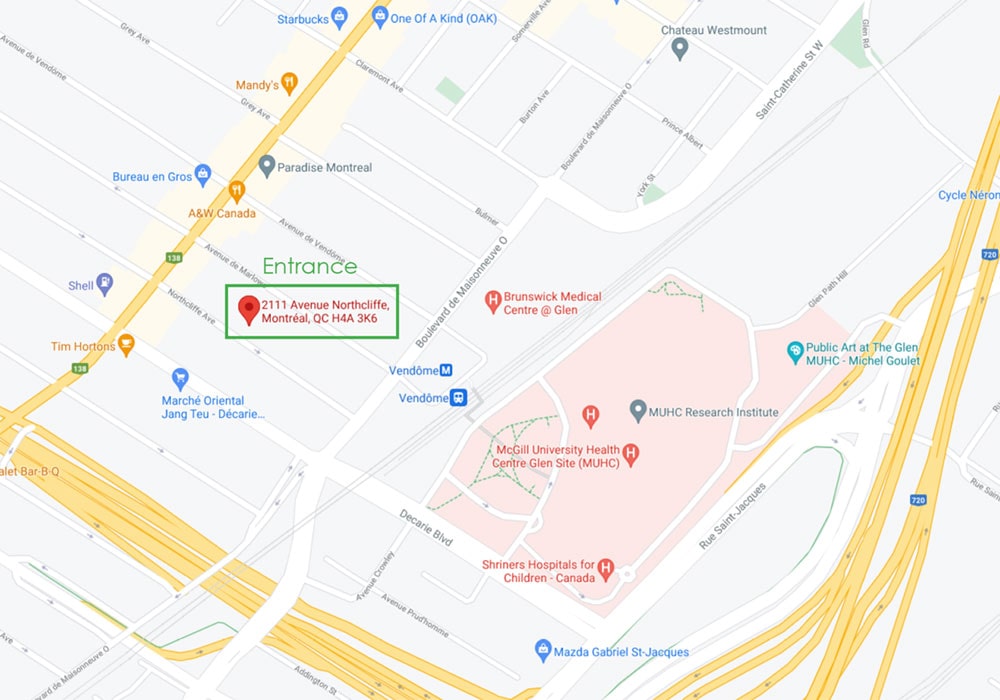
The DOvEEgene team is currently based at the McGill University Health Center, Glen Site and at the Queen Elizabeth Health Complex. The entrance is currently located on 2111 Northcliffe Ave, Montreal, QC H4A 3K6.
Read the Frequently Asked Questions section for more information.